Part B: 13-Mark Questions
1. What are the water quality parameters? Explain their significance.
Water quality parameters are the physical, chemical, and biological characteristics used to evaluate the suitability of water for various purposes.
Physical Parameters- Colour: This is the shade of the water, caused by tiny pieces of organic and inorganic matter.
Significance: Colour is a problem for things like dyeing and laundry. A yellowish colour can mean there is chromium or organic matter, while a yellowish-red colour suggests iron. - Tastes and Odours: This refers to how the water tastes and smells, caused by chemicals (e.g., from algae or rotting plants).
Significance: Bad tastes and odours are not good for industries that make food, drinks, textiles, and paper. - Turbidity and Sediments: This is how cloudy or murky the water is, caused by floating bits like clay, silt, or plant matter.
Significance: High turbidity can affect taste and smell. It also makes it harder to clean the water and is bad for aquatic life because it blocks sunlight.
- pH: This measures how acidic or basic the water is (0-14).
Significance: pH controls how well other substances dissolve. A big change in pH can be a sign of chemical pollution. - Alkalinity: This is the water's ability to neutralize acid, caused by hydroxides, carbonates, and bicarbonates.
Significance: High alkalinity can harm aquatic organisms and cause "caustic embrittlement" in boiler pipes. - Total Dissolved Solids (TDS): This measures all dissolved substances.
Significance: High TDS can make water taste salty and may not be good for drinking or industrial use. - Fluoride: A natural element in groundwater.
Significance: A certain amount is good for dental health. Too little can cause cavities; too much can cause fluorosis. - Arsenic: A poisonous element from nature or industry (farming, mining).
Significance: Long-term arsenic in drinking water can lead to serious health issues, including skin, bladder, and kidney cancer, diabetes, and hypertension. - Chemical Oxygen Demand (COD): A measurement of how much oxygen is needed to chemically break down all impurities.
- Bacterial Impurities: Includes bacteria, fungi, and other tiny living organisms.
Significance: These impurities can cause diseases.
2. Write the detail description about the municipal water treatment process.
The main goal of water treatment is to remove impurities and harmful bacteria to make water safe for use. The process typically involves these main stages:
- Screening: Large, floating materials like leaves and wood are removed by passing the raw water through a screen.
- Aeration: Water is mixed with air to remove unpleasant odours and gases.
- Coagulation and Filtration: Chemicals are added to make small impurities clump together. These clumps settle, and the water is passed through filters (sand, gravel) to remove remaining particles, bacteria, and other impurities.
- Sterilization (Disinfection): This final step destroys any remaining harmful bacteria to make the water safe to drink, commonly done by adding chlorine.
3. What are various boiler troubles and how they are prevented?
Boiler troubles are issues that arise from impurities in the water used in boilers. The main problems are:
Scales and SludgesThese are deposits inside the boiler. Sludges are loose deposits; scales are hard, adherent coatings.
- Prevention: Can be prevented by using chemicals like disodium hydrogen phosphate (Na2HPO4) and sodium aluminate (NaAlO2).
Priming is the production of wet steam. Foaming is the formation of stable bubbles. These are caused by impurities like oil, grease, or high water levels.
- Prevention: Can be controlled by keeping the water level low, controlling steam velocity, and adding anti-foaming agents like synthetic polyamides.
This is the process where the boiler metal becomes brittle due to caustic soda (NaOH).
- Prevention: Can be prevented by using sodium phosphate for water softening (instead of sodium carbonate) and by adding agents like tannin or lignin.
This is the decay of boiler metal, mainly caused by dissolved oxygen and carbon dioxide.
- Prevention: Dissolved oxygen can be removed by adding sodium sulfite (Na2SO3) or hydrazine (N2H4). Dissolved carbon dioxide can be removed by adding ammonium hydroxide (NH4OH).
4. Explain the zeolite softening process and internal conditioning of water.
(i) Zeolite Softening ProcessThe zeolite process softens hard water using zeolite, a hydrated sodium aluminosilicate. Hard water is passed through a cylinder with zeolite. The sodium ions in the zeolite are exchanged for the hardness-causing calcium (Ca2+) and magnesium (Mg2+) ions from the water.
- Regeneration: When the zeolite is used up, it is regenerated by treating it with a strong solution of sodium chloride (common salt).
- Advantages: This process is cheap, the treated water has very low hardness, and it does not produce any sludge.
Internal conditioning is treating water directly inside the boiler to prevent problems.
- Phosphate Conditioning: Adding chemicals like disodium hydrogen phosphate (Na2HPO4) to boiler water. This reacts with hardness salts to form a loose, non-sticky precipitate that can be easily removed.
- Colloidal Conditioning: Adding organic substances (kerosene, gelatin, tannin). These coat small impurities, making them form a loose sludge that is easily removed.
- Sodium Aluminate Conditioning: Adding sodium aluminate (NaAlO2) forms a sticky precipitate that traps impurities and also reacts with magnesium to form a loose precipitate.
5. Explain the demineralization of water by ion exchange process.
The demineralization process (ion exchange) produces pure water free of all dissolved ions (positive and negative). It is more effective than the zeolite process, which only removes hardness ions.
The process has two main steps:
- Cation Exchange: Hard water passes through a cation exchange resin. This resin captures positive ions (like Ca2+ and Mg2+) and replaces them with hydrogen ions (H+), making the water acidic.
- Anion Exchange: The acidic water flows through an anion exchange resin. This resin captures negative ions (like Cl- and SO42-) and replaces them with hydroxide ions (OH-).
The H+ and OH- ions then combine to form pure water (H2O). The resulting water is called demineralized water.
6. Explain in detail Ion exchange process and why it is advantageous over zeolite process.
The demineralization (ion exchange) process uses special resins to remove all dissolved mineral salts from water, making it extremely pure.
How it Works:
- Cation Exchange: Hard water first goes through a cation exchange resin, which captures all positive ions (like calcium and magnesium) and replaces them with hydrogen ions (H+), making the water acidic.
- Anion Exchange: The acidic water then goes through an anion exchange resin, which captures all negative ions (like chloride and sulfate) and replaces them with hydroxide ions (OH-).
The H+ and OH- ions combine to form pure water (H2O).
Why It's Better Than the Zeolite ProcessThe demineralization process is advantageous because:
- Complete Purity: It removes all dissolved ions, while the zeolite process only removes the ions that cause hardness.
- Wider Use: It can treat acidic water, which the zeolite process cannot handle.
- Handles Turbidity: It works even with slightly cloudy water, unlike the zeolite process, where impurities can clog the resin.
7. With necessary diagram, describe the reverse osmosis method for the desalination of brackish water.

Reverse osmosis (RO) is a method used to purify water by forcing it through a special membrane. It's often used to turn brackish or seawater into drinking water.
How It WorksNormally, in osmosis, water flows from a less salty area to a saltier one. In reverse osmosis, a strong external pressure is applied to the salty water. This pressure is greater than the natural osmotic pressure, forcing water molecules to move in the opposite direction—from the salty side to the fresh side, through a semi-permeable membrane.
ResultThe pure water molecules pass through the membrane, leaving the dissolved salts and impurities behind. This process effectively separates the fresh water from the concentrated salt solution (brine).
8. What is break-point chlorination? Explain showing different zones. What are the advantages of break-point chlorination?
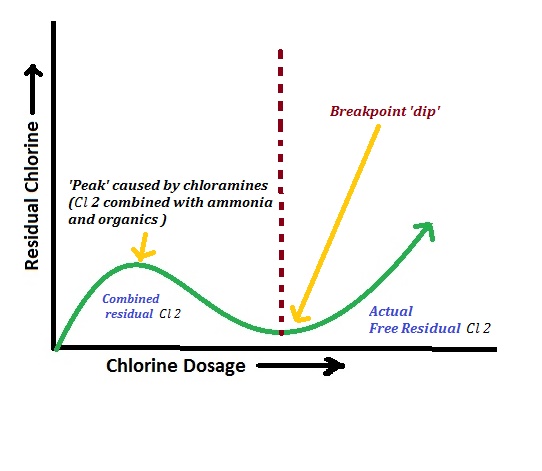
Break-point chlorination is a process where chlorine is added to water until a specific point where all impurities are oxidized, and free chlorine is left behind to act as a disinfectant.
The process is explained in four zones (as seen on a graph):
- Zone AB: Applied chlorine is used to oxidize reducing substances in the water.
- Zone BC: The chlorine forms new compounds with impurities. The amount of combined chlorine increases.
- Point C (Break Point): This is the point where all impurities are completely oxidized. There is no combined chlorine left, and the water is disinfected.
- Zone CD: After the break point, any additional chlorine added remains as a free residual chlorine, which acts as a powerful disinfectant.
The advantages of this process are:
- It completely removes color and odor from the water.
- It destroys bacteria.
- It oxidizes all organic matter and other reducing substances.